Recall of pharmaceutical product in unapproved packages
2009-09-02
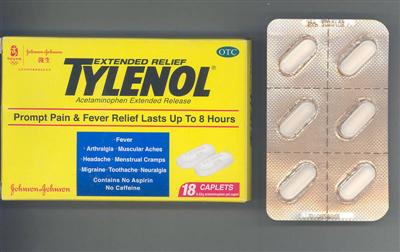
Jacobson Medical (Hong Kong) Ltd, a licensed pharmaceutical wholesaler, is today (September 2) recalling from the retail level a pharmaceutical product, Tylenol Extended Relief Tab 650mg (HK-42812), which has been sold in unregistered packages.
A spokesman for the Department of Health (DH) said the product was an over-the-counter drug. It was imported from China since January this year and used as a pain killer.
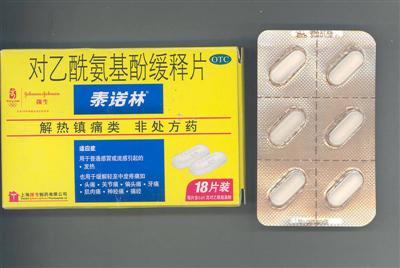
During an investigation conducted today, the company was found to have sold the product in unapproved sales packages with unapproved label information.
The spokesman said under the Pharmacy and Poisons Ordinance, a pharmaceutical product was regarded as registered drug only if the particulars of the product including its package information, corresponded exactly with its registered particulars.
There was no immediate safety and quality concern with the use of the product. Members of the public should stop using the product and seek advice from healthcare professionals as appropriate.
The product was supplied to pharmacies, medicine shops and a chained supermarket.
Healthcare professionals are reminded to stop administering or supplying the product to their clients.
Jacobson Medical (Hong Kong) Ltd has set up a hotline (2596 1821) to answer clients' enquiries.
DH's investigation is on-going.
The spokesman said that sale of unregistered pharmaceutical product was an offence under the Pharmacy and Poison Ordinance. Upon conviction, the maximum penalty is a fine of HK$100,000 and 2 years' imprisonment.
Ends/Tuesday, September 2, 2009